Dentistry / Implantology
The use of the LTT in suspected allergy to dental restorative and implant materials
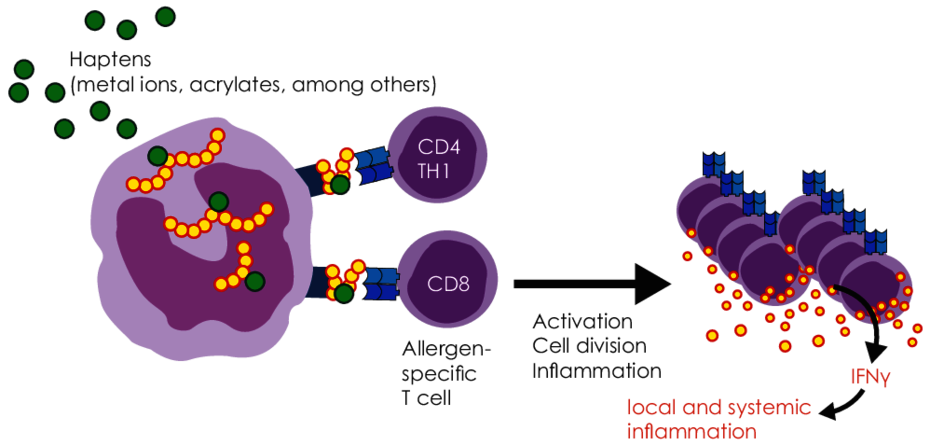
Dental restorative materials, but also incorporated materials in orthopaedic surgery (especially joint replacement implants) or trauma surgery (plates, nails, screws) contain potential allergens. This includes metals and acrylates (plastics) as well as numerous other substances used in dentistry, such as root canal filling materials, cements or ceramics. These foreign materials can act as potential allergens and cause type IV sensitisations.
Metal ions released from alloys and acrylates present as residual monomers are haptens (half-allergens). They bind to proteins in the body and thereby change the structure of these proteins. These modified proteins appear “foreign” (allergenic) to the immune system and thus trigger an immune reaction.
Sensitisation to metals and acrylates are the result of type IV sensitisation, apart from a few exceptions. With this type of allergy, the body produces specific T cells against the corresponding allergen.
In patients who have developed a sensitisation to a metal or acrylate, the immune system responds to repeated contact with this allergen with immune activation (TH1-dominant inflammation). This can manifest in the form of local signs and symptoms, but also trigger systemic immune reactions and augment worsen inflammatory diseases.
Special clinical features of allergy-related material intolerance
Possible local oral manifestations include stomatitis, lichen ruber planus, gingivitis and periodontitis. However, these are not necessarily present as the mucous membranes of the oral cavity show little immunological reactivity. This is understandable when one considers that the oral cavity is the primary point of entrance for foreign antigens (pathogens, food, etc.).
Because of the special immunological situation in the oral mucous membrane, it is not uncommon that local symptoms, such as burning sensation on the tongue or jaw pain and toothache, do not have morphological correlates.
Since immune reactions are principally of systemic nature, numerous general symptoms can occur. These are normally not caused by the dental restorative material, but this can act as a trigger.
These unspecific symptoms include, among others:
headache, migraine, neuralgias, muscle pain, arthralgia, fibromyalgia, paraesthesia, increased tiredness, disturbed sleep, and depressive mood. It is known from clinical case studies that in sensitised patients chronic exposure to metal ions (mercury, gold, nickel, among others) can trigger autoimmunity (chronic arthritis, neurological diseases).
When should testing with the LTT be performed?
There are 2 indications for testing for materials:
- Curative question
After the introduction of foreign material into the body, symptoms appear. Here, the LTT can be used to verify or rule out that these are related to the material.
The LTT answers the question: Is it necessary to change the existing replacement material? - Preventative question
The objective here is to rule out that at the time of introduction of new materials into the body, the patient has an allergic sensitisation to a contact allergen contained in these materials.
The preventive LTT answers the question: What materials can be used or should not be used?
| Please note: Preventive testing should only be perform using the LTT and not with the epicutaneous test, because with latter the patient is exposed to a potential allergen (in the skin) and this by itself can result in (iatrogenic) sensitisation. For this reason, the epicutaneous test guideline of the German Contact Allergy Groupdoes not recommend preventive testing. |
Which materials can be tested for using the LTT?
In principle, the LTT can be used to test for almost any material and material component.
For native materials, a cytotoxic effect or unspecific activating properties have to be ruled out in the laboratory in advance or through parallel testing of control subjects.
For common questions, profiles were developed to test for the known sensitising individual allergens in a standardised way.
LTT profiles
| LTT Metals | 14 standard metals: mercury, copper, silver, tin, ethylmercury, gold, nickel, palladium, chrome, cobalt, molybdenum, aluminium, platinum, cadmium |
| LTT Plastics | TEGDMA, BISGMA, BISDMA, HEMA, methyl methacrylate (MMA), diurethane dimethacrylate (DUDMC), ethylene glycol dimethacrylate, butanediol 1,4-methacrylate, hydroquinone, N,N-dimethyl-4-toluidine, benzoyl peroxide, formaldehyde, phthalate, camphorquinone |
| LTT combination profile (dental check) | Metals: gold, nickel, palladium, chromium, cobalt, platinum, mercury, copper, silver, tin Plastics: methyl methacrylate (MMA), hydroxyethyl methacrylate (HEMA), TEGDMA, BISGMA |
| LTT Gold | Alloys gold + alloy components: gold, silver, platinum, copper, palladium, tin, gallium, indium, iridium, rhodium, tantalum, ruthenium |
| LTT Amalgams | amalgam components and organic mercury compounds: mercury, copper, silver, tin, ethyl mercury, phenyl mercury, methyl mercury |
| LTT Root Canal Filling Materials | raw gutta-percha, Balsam of Peru, eugenol, PDMS, silicone oil, bismuth oxide, turpentine oil, colophony, triethanolamine, peanut oil, paraformaldehyde, bisphenol A, epichlorohydrin |
| LTT Ceramic and Cements | vanadium, aluminium, titanium, cobalt, chromium, barium, silicon, cerium, boron, manganese, antimony, phosphate cement (Harvard), glass ionomer base cement (Ketac-Bond) |
| LTT-Titanium Materials | Titanium + Alloy Components: titanium dioxide, nickel, vanadium, aluminium |
| LTT Endoprosthetics | chromium, cobalt, molybdenum, nickel, titanium, vanadium, niobium, aluminium, zirconium (IV) oxide, methyl methacrylate, N,N-dimethyl-p-toluidine, benzoyl peroxide, hydroquinone, gentamicin |
| LTT Native Material | testing for native materials submitted together with blood samples |
For titanium intolerance testing, please refer here.
Is it also possible to tested for individual materials?
With the LTT Native Material, there is also the option of testing materials which are submitted by the physician, dentist or the dental lab together with the blood sample to the laboratory. This procedure has proven to be successful particularly with complex materials like cements, composites, prosthesis materials, bone graft substitute materials, root canal filling materials (also pins) as well as intraoral harvested metal and plastic chips and shavings.
If materials have tested negative in the LTT, it is ruled out that there is sensitisation to substances contained in these materials whether they are declared or not. Materials which have tested positive in the LTT should under no circumstances be placed in the patient's body, because the patient has a type IV sensitisation to at least one of the components.
note: A selection of native materials (commonly used composites etc.) is available in the laboratory. Please call us under +49 30 77001-220 and we will send you this list per fax.
Are any cost-effective combined profiles available?
A combined profile (Dental-Check) was compiled in particular for preventive testing in dentistry which covers the most important metals and acrylates. Although this profile is not comprehensive, it covers the most important contact allergens, i.e. those allergens in the test profiles LTT Metals and LTT Plastics which most frequently tested positive in the past.
| Metals | gold, silver, palladium, copper, tin, cobalt, chromium, nickel, platinum, and mercury |
| Acrylates | hydroxyethyl methacrylate (HEMA), methyl methacrylate (MMA), triethylene glycol dimethacrylate (TEGDMA), BISGMA |
The LTT Dental Check thus includes the problem areas:
- gold alloys (Au, Pt, Pd, Ag, Cu, Sn)
- NEM alloys (Cr, Co, Ni)
- amalgam (Hg, Ag, Sn, Cu)
- acrylate containing composites and cements (HEMA, TEGDMA, BISGMA)
- acrylic resins for prostheses (MMA)
Is there a special profile available for testing ahead of joint replacement surgery?
Especially for preventive testing a combined profile was compiled, which includes, besides the metals potentially contained in implants, the components of cements. Among these components are acrylates, the polymerisation initiators benzoyl peroxide and hydroquinone, as well as gentamicin which is found in most cements.
What about titanium?
Attention:A negative LTT result for titanium rules out only an allergic sensitisation which is very rare. To find out whether the patient has an increased propensity for inflammation on exposure to titanium oxide particles, especially in preventive questions, it is necessary to investigate any genetic predisposition for inflammation (TNF-α, IL-1α, IL-1β and IL-1RA) and to perform the titanium stimulation test. |
Please request the “Titanium Intolerance” diagnostic information sheet from our laboratory.
What are the consequences of a positive LTT result?
A positive test result in the LTT (but also in skin testing) indicates an existing sensitisation to the respective allergen. The material that tested positive should not be used in future treatments.
However, no allergy test can prove that there is a causal link to existing symptoms. Thus, with a proven sensitisation it must be carefully considered whether the respective problem material should be removed and replaced with a different material.
Here, the severity of the symptoms is critical, by no means a positive test result alone. Other sources of exposure should be eliminated primarily or at the same time (detailed information to other sources of exposure is available with every positive LTT result).
When is the Multi-Element Analysis/MEA of the metals advisable?
In particular with dentistry-related queries, sometimes the question arises whether a confirmed sensitisation to a certain metal has therapeutic consequences.
This is only the case when the patient has been exposed to the respective metal through existing restorative dental work. In some cases this is not clear, for example, when it is not known whether older existing gold alloys contain copper, silver or palladium or whether there is a potential exposure to nickel or chromium from a (usually not declared) point of soldering in the dental work.
For these cases, the multi-element analysis in the saliva can be a useful addition to the diagnostic work-up.
If the saliva test (morning saliva or chewing gum test) shows detectable values for the metal that tested positive in the LTT, the source of the substance has to be investigated and the material has to be removed, if possible. If a patient has a type IV sensitisation to a metal, even mild elevations are of relevance which is in contrast to the toxicological perspective where only significantly increased metal levels in saliva are regarded as clinically important.
Below a sample case report of a 44-year-old female patient who tested positive in the LTT for a sensitisation to aluminium, nickel and palladium. The multi-element analysis of her morning saliva identifies her existing restorative dental work as the source of her significant exposure to gold, silver and also palladium. In so far, a removal of the apparently palladium-containing gold alloys is indicated even though there is no allergic sensitisation to gold itself.
The patient is not exposed to aluminium and nickel, at least not by restorative dental work. Here, the source of exposure must be searched elsewhere (food, toner exposure etc.).
Are there any allergies against root canal filling materials?
Yes, gutta-percha and also the various available root canal sealers on the market often contain one or more substances that give rise to concern. These include very potent allergens such as Balsam of Peru, bisphenol A, paraformaldehyde and silicone, peanut or turpentine oil.
For curative, but even more so for preventive queries, the profile LTT Root Canal Filling Materials is indicated. Apart from gutta-percha, this profile contains those ingredients of commonly used root canal sealers that are known to have caused problems.
In case of a positive test result, a material must be selected which does not contain the respective substance.
Allergy testing for gutta-percha uses natural raw gutta-percha. Especially with preventive queries it can be useful to test the specific gutta-percha product to be used, because, for example, synthetically produced gutta-percha or processed natural raw materials do not necessarily contain any longer all allergen components of the raw gutta-percha.
Effector cell typing is never a substitute for the LTT in preventive testing
In individual cases with proven sensitisation in the LTT and uncertain clinical significance, it is possible to confirm or invalidate the causal relationship between sensitisation and clinical manifestation by subsequently performing an effector cell typing.
In effector cell typing, the effector cells involved in the immune reaction are closer characterised (typed) by the allergen-stimulating cytokine reactions. Differentiation occurs into IFN-γ secreting TH1 effector cells, into IL-10 releasing regulatory T cells and into memory cells without current effector function which (only) release IL-2. Especially if no previous LTT Result is available, the test profile is complemented by IL-2 and TNF-α.
However, effector cell typing is no alternative to LTT, especially in preventive testing, because it cannot detect present latent allergies with certainty. In fact, there are memory lymphocytes which do not release cytokines.
A negative effector cell typing result should not lead to the decision to introduce the respective material into the body, because with permanent exposure to the respective allergen it is likely that the effector reaction develops into a cytotoxic TH1 immune reaction.
Please contact us for further information about effector cell typing.
Is the LTT a validated laboratory method?
At present, the LTT is the only laboratory method to test for specific cellular sensitization. By further development in cell culture technique and analytical methods the test has emerged to a reproducible and highly sensitive method for medical diagnostic testing.
A positive reaction in the LTT indicates the presence of allergen-specific lymphocytes (memory cells) in the patient’s blood.
Since 2004, the LTT has been accredited by DAkkS according to DIN EN ISO 17025 and its sensitivity and specificity are monitored by ring and comparative testing.
Although today the LTT is generally professionally recognized as a method to detect sensitisation to medications and beryllium, the LTT’s validity in metal or plastics allergies is still discussed controversially.
This is insofar surprising, as the methodology does not differ between the various areas of use.
The reason for the differentiated view is unfortunately rather related to differences in the perception of the problem areas drug allergy and material intolerance.
Whereas drug allergy is not considered controversial, very conservative allergists see the metal and acrylate allergies as rare conditions and think that the reported symptoms are rather fully or partially of psychosomatic origin.
In this point in particular, the LTT has provided valuable services by helping to provide objective parameters in relation to the symptoms.
Literature
- von Muris J. et al.(2009) Reactivity to sodium tetrachloropalladate (Na2[PdCl4]) compared to PdCl2 andNiCl2 in lymphocyte proliferation tests. Allergy.; 64: 1152-6.
- Thomas P. et al. (2009) Increased metal allergy in patients with failed metal-on-metal hip arthroplasty and peri-implant T-lymphocytic inflammation. Allergy.; 64: 1157-65.
- Vamnes JS et. al.(1999) In vitro lymphocyte reactivity to gold compounds in the diagnosis of contact hypersensitivity. Contact Dermatitis.;41:156-60.
- Thomas P et al. (2006) Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: case report and review of the literature. Contact Dermatitis.;55: 199-202.
- Rasanen L. Et al. (1992) Diagnostic value of the lymphocyte proliferation test in nickel contact allergy and provocation in occupational coin dermatitis. Contact Dermatitis.; 27: 250-4.
- von Baehr V et al. (2001) Improving the in vitro antigen specific T cell proliferation assay: the use of interferon-alpha to elicit antigen specific stimulation and decrease bystander proliferation. J Immunol Methods.;251: 63-71.
- Bartram F. et al. (2006) Stellungnahme des Deutschen Berufsverband der Umweltmediziner zur Bedeutung von Epikutantest und Lymphozytentransformationstest für die Diagnostik von Typ IV-Sensibilisierungen. Journal of Laboratory Medicine ; 30 : 101-106).
- Everness KM. Et al. (1990) The discrimination between nickel-sensitive and non-nickel-sensitive subjects by an in vitro lymphocyte transformation test. Br J Dermatol.;122: 293-8.
- Lebeau J. (2007) Use of the Beryllium Lymphocyte Proliferation Test (BeLPT) for screening. J Occup Environ Med.;49: 357-8.
- Cederbrant K. et al. (1997) In vitro lymphocyte proliferation as compared to patch test using gold, palladium and nickel. Int Arch Allergy Immunol.;112: 212-17.
- Rustemeyer T. et al. (2004) Analysis of effector and regulatory immune reactivity to nickel. Clin Exp Allergy; 34: 1458-66.
- Hallab NJ. et al. (2004) Lymphocyte transformation testing for quantifying metal-implant-related hyper-sensitivity responses. Dermatitis.;15: 82-90.
- Hallab NJ. et al. (2005) Lymphocyte responses in patients with total hip arthroplasty. J Orthop Res. ;23: 384-91.
- Stange AW. Et al. (2004) , Furman FJ, Hilmas DE. The beryllium lymphocyte proliferation test: Relevant issues in beryllium health surveillance. Am J Ind Med.;46: 453-62.
- Milovanova TN. et al. (2007) Comparative analysis between CFSE flow cytometric and tritiated thymidine incorporation tests for beryllium sensitivity. Cytometry B Clin Cytom.;72: 265-75.
- Moneret-Vautrin D.A. (2004) Allergy to nickel in dental alloys. Europ. Annals of Allergy and Clin Immunol; 36: 311-2.
- piewak R, Moed H, et al. (2007) Allergic contact dermatitis to nickel: modified in vitro test protocols for better detection of allergen-specific response. Contact Dermatitis.; 56: 63-9.
- Valentine-Thon E. et al. (2006) LTT-MELISA(R) is clinically relevant for detecting and monitoring metal sensitivity. Neuro Endocrinol Lett. ;27: 17-24.
- Valentine-Thon E et al. (2003) Validity of MELISA for metal sensitivity testing. Neuro Endocrinol Lett.;24 : 57-64.
- Lindemann M. et al. (2008) Detection of chromium allergy by cellular in vitro methods. Clin Exp Allergy.; 38: 1468-75.
- Räsänen L et al. (1991) Lymphocyte proliferation test as a diagnostic aid in chromium contact sensitivity. Contact Dermatitis.; 25: 25-9.
- Deubner DC et al. (2001) Variability, predictive value, and uses of the beryllium blood lymphocyte proliferation test (BLPT). Appl Occup Environ Hyg.;;16: 521-6.
- Minang JT et al. (2006) Nickel, cobalt, chromium, palladium and gold induce a mixed Th1- and Th2-type cytokine response in vitro in subjects with contact allergy to the respective metals. Clin Exp Immunol.; 146: 417-26.
- Stange AW. Et al. (2004) The beryllium lymphocyte proliferation test: Relevant issues in beryllium health surveillance. Am J Ind Med.;46: 453-62.